Circular mRNA
In early 2020, the pneumonia pandemic (COVID-19) caused by the novel coronavirus (SARS-CoV-2) began to spread globally and has continued to this day. Unfortunately, the situation of the epidemic is still very serious today. According to the World Health Organization (WHO), more than 500 million people have been infected worldwide, and the cumulative death toll has exceeded 6 million. The data released by the WHO recently revealed that the number of excess deaths related to the virus worldwide was approximately 15 million in 2020 and 2021.
mRNA vaccines have emerged as the most promising and effective platforms against multiple diseases, including the COVID-19 pandemic. (However, urgent need to develop stable, safe, and effective mRNA vaccines has been highlighted by the instability of mRNA by nature), the various side effects associated with delivery systems, and the constant emergence of virus mutants. However, the inherent instability of mRNA, the variety of side effects associated with delivery systems, and the continuous emergence of viral mutants highlight the urgent need to develop stable, safe, and effective mRNA vaccines
(On May 13, 2022, SuZhou CureMed Biomedical Technology Co., Ltd. (hereinafter referred to as CureMed) published a paper on the preprint platform bioRxiv entitled: Delivery of Circular mRNA via Degradable Lipid Nanoparticles against SARS-CoV-2 Delta Variant [1]). On May 13, 2022, one paper entitled: Delivery of Circular mRNA via Degradable Lipid Nanoparticles against SARS-CoV-2 Delta Variant [1] published by SuZhou CureMed Biomedical Technology Co., Ltd. (hereinafter referred to as CureMed) on the preprint platform bioRxiv.
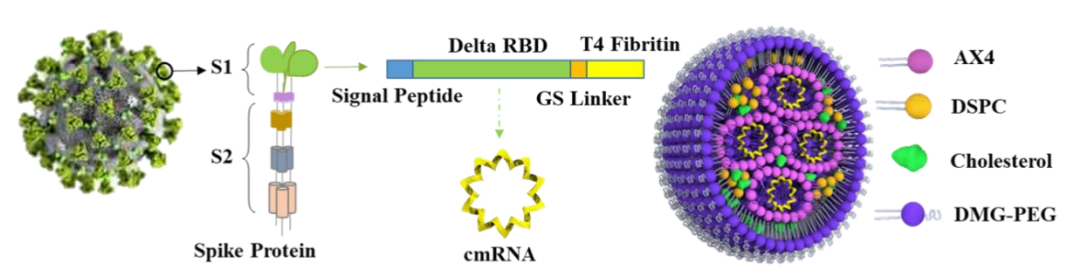
The study reported a novel circular mRNA vaccine, cmRNA-1130, (delivering the circular mRNA (cmRNA) of the receptor binding domain (RBD) of encoded SARS-CoV-2 spike by novel degradable lipid nanoparticles (LNPs). This circular mRNA vaccine induces robust immune activation, including potent and sustained neutralizing antibodies, as well as CD4+ T and CD8+ T cells). a circle mRNA (cmRNA) encoding the receptor binding domain (RBD) of SARS-CoV-2 spike protein delivered by by novel degradable lipid nanoparticles (LNPs). This cmRNA vaccine can induce robust immune activation including effcetive and sustained neutralizing antibodies and CD4+ T and CD8+ T cells.
The AX4-LNP used in this study showed faster hepatic and splenic clearance compared to the commercial MC3-LNP. In addition, the cmRNA-1130 vaccine maintained excellent stability at 4°C and repeated freeze-thaw cycles for 6 months.
In general, this study demonstrated that mRNA vaccines based on circular mRNA and biodegradable AX4-LNP can serve as a promising platform for the prevention and treatment of various diseases.

In nature, circular RNAs (circRNAs) are ubiquitous in fungi, plants, insects, fish and mammals, and even in the genomes of some viruses by nature. Unlike linear mRNA, circRNAs are highly stable because of their covalently closed ring structure which protects it from exonuclease-mediated degradation.
Therefore, the circular mRNA becomes a new option for vaccine. Since 2021, a number of domestic and foreign companies in the circRNA field have obtained financing, such as Orna Therapeutics and Laronde, especially the latter receives 440-million-dollar Series B round financing round from Flagship which has incubated Moderna, ignited the competetion in circRNA field. In addition, domestic engagers CirCode and Therorna have also successively obtained hundreds of millions of finances.
In November 2021, CureMed published a paper on the preprint platform bioRxiv [2], which demonstrated for the first time in a mouse model that direct intratumoral injection of a mixture of cytokines encoded by cyclic mRNA could regulate intratumoral systemic effects of anti-tumor immune response and enhanced the tumor suppressive effect of anti-PD-1 mAb. CureMed plans to advance an investigator-initiated trial (IIT) in the near future to realize the first application of circular mRNA in human.

In April 2022, CureMed published a paper on the preprint platform bioRxiv [3], reported the development of a circular mRNA-based novel target protein degradation platform. CureMed used the bioPROTAC molecules encoded by circular mRNA to degrade target proteins and named such platform as RiboPROTAC. With this technique, the pattern protein GFP and the cellular endogenous protein PCNA were successfully degraded. PCNA degraders encoded by circular mRNAs also exhibited significant anti-tumor effects in vivo.
This is the first proof-of-concept for RiboPROTAC as an mRNA therapeutic. RiboPROTAC will be a new and powerful technology for targeting undruggable targets. Compared with small-molecule PROTAC, RiboPROTAC has the advantages of rapid development speed, high binding specificity to target proteins, and self-expression of E3 ubiquitin ligase, which is not affected by the abundance and mutation of E3 ubiquitin ligase in host cells. Compared with protein-based bioPROTAC, RiboPROTAC has superiority with systemic or local delivery capacity, longer intracellular retention time, and simpler preparation process. Based on the RiboPROTAC platform, more undruggable targets will become druggable.

In this latest paper that launched in May, CureMed expanded its circular mRNA technology to the field of COVID-19 vaccines.
In recent years, lipid nanocarriers such as LNPs have shown great potential for RNA delivery, demonstrating high safety and efficacy in clinical trials.
With the widespread use of mRNA vaccines, it is found in many studies that LNPcarriers used to deliver mRNA may cause inflammation, leading to side effects and potential toxicity. Therefore, it is necessary to develop novel LNP delivery platforms with better biodegradability and biocompatibility.
The lipid components of typical LNPs usually include four types: cationic lipids, helper lipids, cholesterol and PEGylated lipids.
The research team designed a series of ionizable cationic lipids. Among them, the cationic lipid AX4 (patented) with high expression and rapid metabolism was obtained from in vivo experiments in mice. With microfluidic technology, AX4 was combined with DSPC, cholesterol and DMG-PEG and mRNA to produce LNP, of which the particle size distribution was uniform with the average particle size 85nm and the PDI 0.10. Compared to MC3-LNP, AX4-LNP showed faster liver and spleen elimination via intravenous administration, which helped eliminate potential toxic side effects and facilitated the feasibility of repeated dosing.
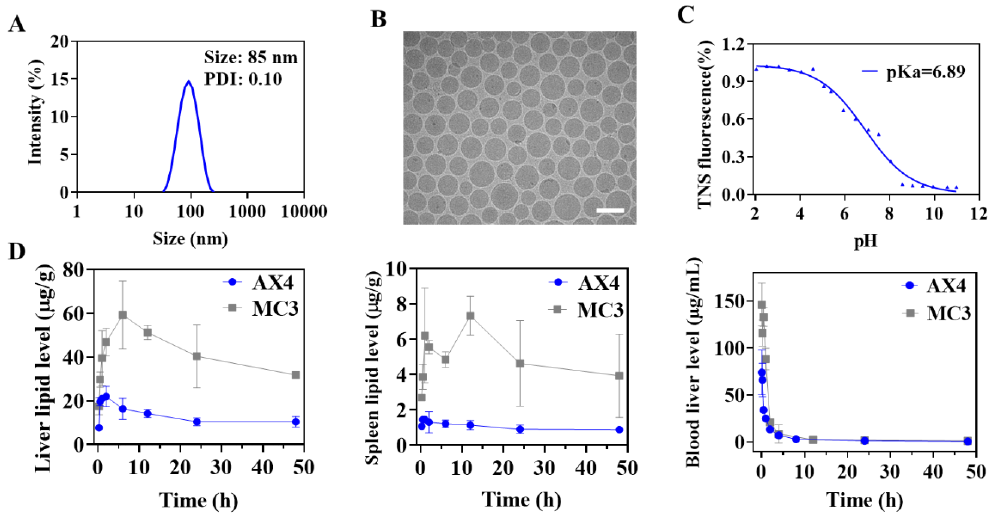
Compared with the commercial cationic lipid MC3, AX4 shows higher expression effect, faster metabolism, high liver targeting, and good thermal stability; Moreover, the synthesis route is simple, and the production of kilogram GMP grade AX4 has been realized. (and the simple synthesis route has allowed the kilogram-scale GMP-grade AX4 production.) Moreover, (the AX4 GLP animal safety assessment test)the GLP animal safety assessment test of AX4 will also be completed in July this year, followed by an application (for) of the US FDA DMF number.
The research team then developed a circular mRNA vaccine, (namely)named cmRNA-1130, (against)which targets the Delta mutant of the novel coronavirus. (It delivers the circular mRNA (cmRNA) expressing the receptor binding domain (RBD) of the Delta mutant spike protein with AX4-LNP.) cmRNA-1130 expressing the receptor binding domain (RBD) of the Delta mutant spike protein was delivered by AX4-LNP.
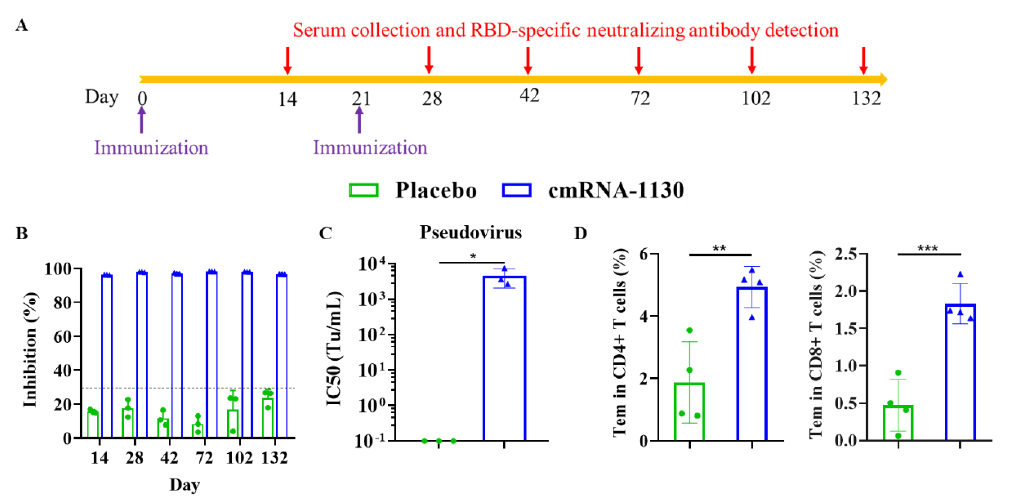
The circular mRNA vaccine successfully induced robust neutralizing antibody (NAb) responses and T cell responses in mice. The induced NAbs showed significant neutralizing activity against Delta pseudovirus and could last for at least 132 days in mice, indicating that the protective effect of the vaccine is not only efficient but also durable.
In addition, considering the stability of the circular mRNA itself, the vaccine can be stored stably at 4℃ for more than 6 months without RNA modification, (and) Furthermore, It can also remain stable after 6 repeated freeze-thaw cycles. Compared with linear mRNA vaccines, the circular mRNA type is more convenient to store, transport and distribute, and has great clinical potential as a preventative vaccine to restrain COVID-19 pandemic.
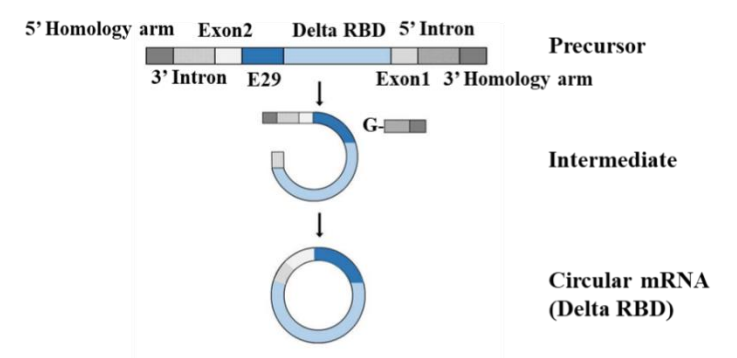
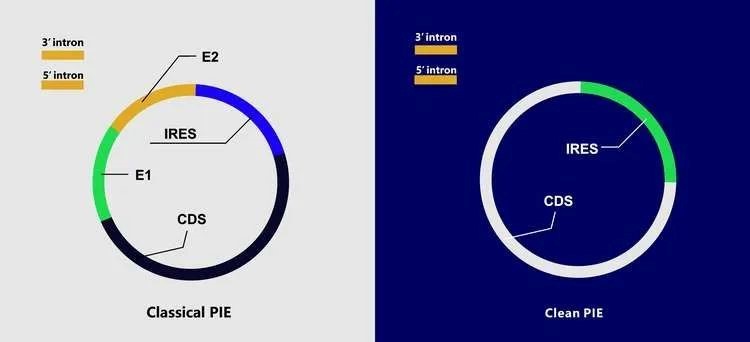
(In circular RNA research, the effectivity of circularization is a difficulty).Tthe effectivity of circularization is a difficulty in circular RNA research, CureMed has developed a novel Clean PIE system for circular RNA preparation in vitro, which can avoid introducing extra exon sequences during the circularization process. It reduces the immunogenicity of circular RNA by improving the sequence accuracy of circular RNA molecules, and can provide a circularization effectivity that more (greater) than 90%. This circularization system has been patented. It is promising in the fields of nucleic acid vaccines, protein replacement, and gene therapy.
With its highly stable structure, circular mRNA can be regarded as the 2.0 version of mRNA. It will bring far-reaching changes to the field of biotechnology, make the (early development of drugs) pre-drug development more efficient and economical, and at the same time more technically feasible, and improve the success rate of drug development.
The circular mRNA ecosystem created by CureMed includes circular mRNA Clean PIE circularization frame, IRES elements, regulatory elements, codon optimization, CMC process development, indications, target applications, etc. The design of such elements brings drug development into the era of modular programmable drugs.
At the same time, CureMed has established a huge library of LNP cationic lipid compounds, while only a small part of was reported in the paper, of which the AX4-LNP is suitable for researches in mRNA vaccines and some liver diseases with its high liver targeting attribution. For different indications and application scenarios, CureMed is developing a variety of organ- or tissue-specific cationic lipids to achieve specific targeting on the spleen, lung, hematopoietic stem cells, central nervous system, etc.
Based on the above research, CureMed has established a R&D pipeline that covers tumor immunity, protein replacement, cell therapy and infectious disease vaccines, laying the stress on tumor immunity, where researches on IIT and IND are progressing. The team has rich experience in CAR-T cell research and is undertaking CAR-NK cell research based on circular mRNA. Considering the constant mutation of the novel coronavirus, CureMed is exploring a (universal)general vaccine based on circular mRNA to deal with the various mutant strains.
Paper links:
1.https://www.biorxiv.org/content/10.1101/2022.05.12.491597v2
2.https://www.biorxiv.org/content/10.1101/2021.11.01.466725v1
3.https://www.biorxiv.org/content/10.1101/2022.04.22.489232v1
